Both our hydrogen storage and hydrogen compression solutions are based on a reversible chemical reaction of hydride-forming metals or alloys with hydrogen gas resulting in the formation of metal hydrides (MH)
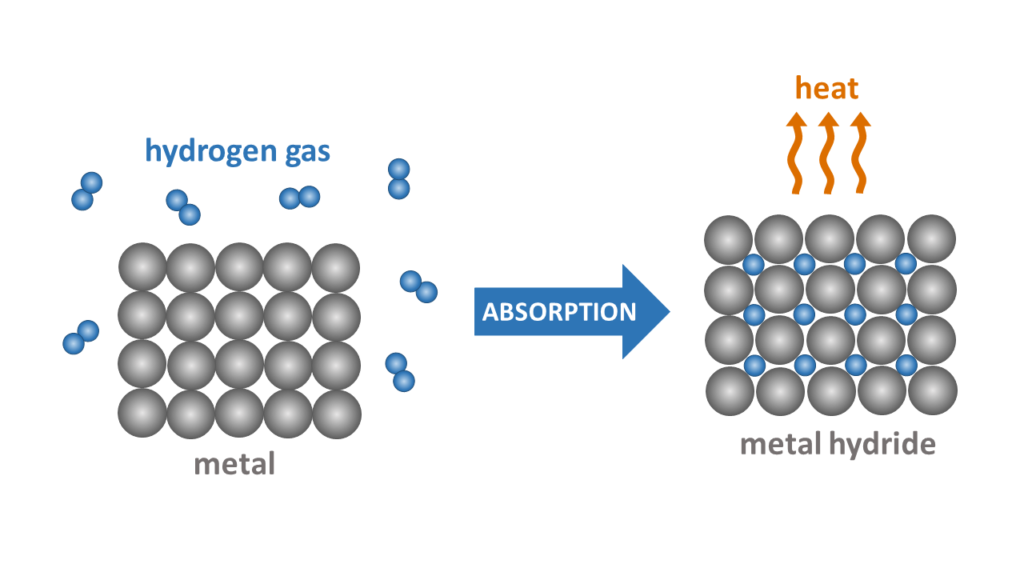
Exothermic hydrogen absorption reaction evolves heat, while hydrogen desorption from the metal hydride is an endothermic process causing cooling of the system. The direction of this chemical reaction depends on both temperature and pressure of the system
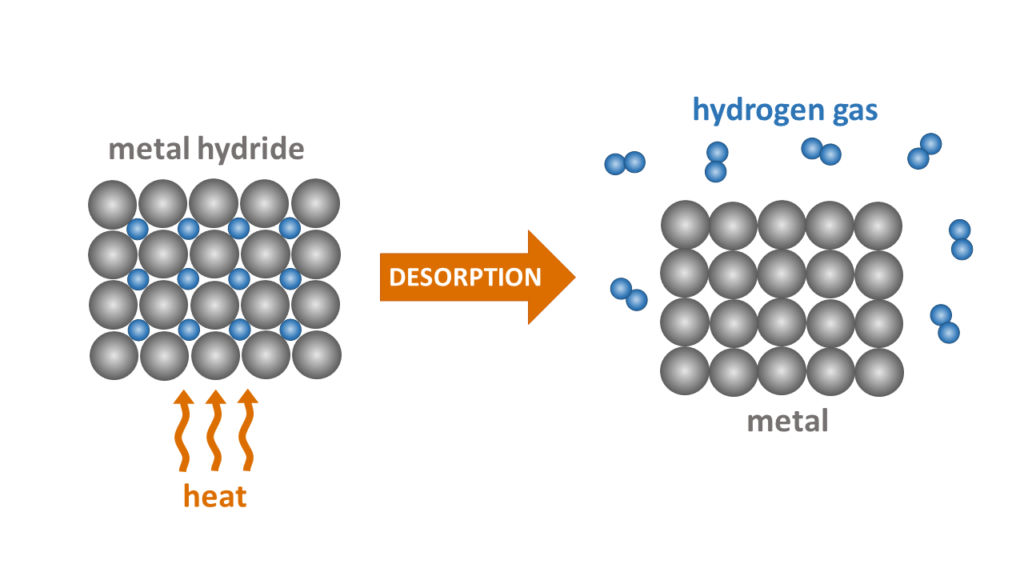
Metal hydrides are very attractive for storing hydrogen in solid state in safe and compact way due to a very high volumetric density of atomic hydrogen accommodated in the interstitial sites of crystal lattice. Hydrogen storage in metal hydrides is intrinsically safe and benefits from avoiding use of compressed hydrogen gas and energy inefficient and potentially unsafe liquid H2. Hydrogen density in our MH storage systems near ambient temperature and low pressure (10-30 bar) is equivalent to density of compressed hydrogen at 1000 bar
Our metal hydride hydrogen compressor has many advantages over conventional compressors (such as the diaphragm and piston compressors) as this thermal compressor has no moving parts, potentially low maintenance cost and compresses the hydrogen in a silent and vibrationless manner. The operation cost can be minimized if waste heat is available as the system utilize thermal energy for the compression work
